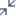Eligible patients may pay as little as $4
per Rx with the Co-Pay Savings Card.†

SAVE ON LYRICA
Offer Terms And Conditions:
By using the Co-Pay Savings Card, you acknowledge that you currently meet the eligibility criteria and will comply with the following terms and conditions:
Patients are not eligible to use this card or participate in this program if they are enrolled in a state or federally funded insurance program, including but not limited to Medicare, Medicaid, TRICARE, Veteran Affairs healthcare, a state prescription drug assistance program, or the Government Health Insurance Plan available in Puerto Rico (formerly known as “La Reforma de Salud”). Patient must have private insurance. Offer is not valid for cash-paying patients. The value of this card is limited to $250 per month per prescription (“offering period”) or the amount of your co-pay, whichever is less (maximum annual savings of $3000). This program is not valid when the entire cost of your prescription drug is eligible to be reimbursed by your private insurance plan or other private health or pharmacy benefit programs. You must deduct the value received under this program from any reimbursement request submitted to your private insurance plan, either directly by you or on your behalf. You are responsible for reporting use of this program to any private insurer, health plan, or other third party who pays for or reimburses any part of the prescription filled using the program, as may be required. You should not use the program if your insurer or health plan prohibits use of manufacturer co-pay cards. You must be 18 years of age or older to accept this offer. This offer is not valid where prohibited by law.
For Massachusetts residents:This co-pay offer is not valid if an A/B generic is available for Massachusetts residents whose prescriptions are covered in whole or in part by third-party insurance.
For California residents:This co-pay offer is not valid if a generic is available for California residents whose prescriptions are covered in whole or in part by third-party insurance.
Please check with your healthcare professional or insurer to confirm eligibility. This offer cannot be combined with any other savings, free trial or similar offer for the specified prescription. The co-pay card will be accepted only at participating pharmacies. The co-pay card is not health insurance. Offer good only in the US and Puerto Rico. The co-pay card is limited to one per person during this offering period and is not transferable. A co-pay card may not be redeemed more than once per offering period per patient. No other purchase is necessary. Data related to your redemption of the co-pay card may be collected, analyzed, and shared with Viatris, for market research and other purposes related to assessing Viatris’s programs. Data shared with Viatris will be aggregated and de-identified; it will be combined with data related to other co-pay card redemptions and will not identify you. Viatris reserves the right to rescind, revoke, or amend this offer without notice. No membership fee. For more information, visit our website www.LYRICA.com, call 1-866-954-1475, or contact us at 2250 Perimeter Park Drive, Suite 300, Morrisville, NC 27560.
Offer expires 12/31/2024.
For reimbursement when using a nonparticipating pharmacy/mail order:Pay for prescription, and mail copy of original pharmacy receipt (cash register receipt NOT valid) with product name, date, and amount circled to: Co-Pay Savings Card, 2250 Perimeter Park Drive, Suite 300, Morrisville, NC 27560. Be sure to include a copy of the front of your activated Co-Pay Savings Card, your name and mailing address.
Mobile Program Terms And Conditions
- By opting into the Viatris LSAVINGS Mobile program (“Program”), in which you can receive your Co-Pay Savings Card via text, you consent to receive approximately 5 text messages and/or push notifications per month from Viatris Inc. Consent is not a condition of purchase or use of any Viatris product or service. Such messages may be marketing or non-marketing messages and may include, for example, refill reminders, fill confirmation, website information, etc. T-Mobile is NOT liable for delayed or undelivered messages.
- To stop receiving text messages, text STOP to 597422. DOING SO WILL ONLY OPT YOU OUT OF THE LSAVINGS MOBILE PROGRAM; you will remain opted into any other Viatris Inc. text message program(s) to which you separately opted in. You may unsubscribe from the digital wallet message Program at any time by disabling push notifications or removing the digital wallet pass from your device for digital wallet programs.
- To request more information or to obtain help, text HELP to 597422. You can also call customer service at 1-877-822-7855.
- You represent that you are the account holder for the mobile telephone number(s) that you provide to opt into the texting Program. You are responsible for notifying Viatris Inc. immediately if you change your mobile telephone number. You may notify Viatris Inc. of a number change by re-enrolling in the Program.
- Message and data rates may apply to each text message sent or received in connection with the texting Program, as provided in your mobile telephone service rate plan, in addition to any applicable roaming charges. Charges are both billed and payable to your mobile service provider or deducted from your prepaid account. Viatris Inc. does not impose a separate fee for sending text messages.
- Data obtained from you in connection with this Short Message Service (SMS) texting program may include your telephone number; your carrier’s name; and the date, time, and content of your messages. Viatris Inc. may use this information to contact you and to provide the services you request from us.
- You understand that data obtained from you in connection with your registration for, and use of, the Program may include, for example, your phone number, related carrier information, device information, and elements of pharmacy claim information. This data may be used to administer this Program and to provide Program benefits such as savings offers, information about your prescription, refill reminders, as well as Program updates and alerts sent directly to your device. Please read our full corporate Privacy Policy, which is incorporated by reference into these terms.
- In addition to the data use practices described in the Privacy Policy, we may send you offer-related push notifications when your device is in the physical proximity of your pharmacy or healthcare provider. This is done through geofencing technology, which is built into your device. Your device’s location will not be known or tracked by Viatris or its service providers. Nonetheless, you may opt out of geofencing and receiving these notifications at any time by (1) disabling location services for your digital wallet app in your device’s settings, (2) disabling notifications (i.e., automatic updates) within the digital wallet app, or (3) removing the eCard from your digital wallet by selecting “Remove Pass” within the digital wallet app.
- Viatris Inc. will not be liable for any delays in the receipt of any SMS messages, as delivery is subject to effective transmission from your network operator.
- The service is available only on these US participating mobile carriers: Verizon Wireless, Sprint, Nextel, Boost Mobile, T-Mobile, AT&T, Alltel, ACS Wireless, Bluegrass Cellular, Carolina West Wireless, Cellcom, Cellular One of East Central Illinois (ECIT), Cincinnati Bell, Cricket Wireless, C Spire Wireless, Duet IP (AKA Max/Benton/Albany), Element Mobile, Epic Touch, GCI Communication, Golden State Cellular, Hawkeye (Chat Mobility), Hawkeye (NW Missouri Cellular), Illinois Valley Cellular (IVC), Inland Cellular, iWireless, Keystone Wireless (Immix/PC Management), MetroPCS, Mobi PCS, Mosaic Telecom, MTPCS/Cellular One (Cellone Nation), Nex-Tech Wireless, nTelos, Panhandle Telecommunications, Pioneer, Plateau, Revol Wireless, Rina-Custer, Rina-All West, Rina-Cambridge Telecom Coop, Rina-Eagle Valley Comm, Rina-Farmers Mutual Telephone Co, Rina-Nucla Nutria Telephone Co, Rina-Silver Star, Rina-South Central Comm, Rina-Syringa, Rina-UBET, Rina-Manti, Simmetry Wireless, South Canaan (Cellular One of NEPA), Thumb Cellular, Union Wireless, United Wireless, U.S. Cellular, Viaero Wireless, Virgin Mobile, and West Central Wireless (includes Five Star Wireless).
- You agree to indemnify Viatris Inc. and parties texting on its behalf in full for all claims, expenses, and damages related to or caused in whole or in part by your failure to notify us if you change your telephone number, including but not limited to all claims, expenses, and damages related to or arising under the Telephone Consumer Protection Act.
- Viatris Inc. may suspend or terminate your receipt of text messages if it believes you are in breach of these SMS Terms and Conditions. Your receipt of text messages is also subject to termination in the event that your mobile telephone service terminates or lapses. Viatris Inc. reserves the right to modify or discontinue, temporarily or permanently, all or any part of the text messaging services you receive, with or without notice.
- Viatris Inc. may revise, modify, or amend these SMS Terms and Conditions at any time. Any such revision, modification, or amendment shall take effect when it is posted to Viatris Inc.’s website. You agree to review these SMS Terms and Conditions periodically to ensure that you are aware of any changes. Your continued consent to receive text messages will indicate your acceptance of those changes.
E-mail Terms And Conditions
By agreeing to the terms of LSAVINGS E-mail program (the “E-mail Program”), you consent to receive e-mail messages on behalf of Viatris. Consent is not a condition of purchase or use of any Viatris product or service.
Data obtained from you in connection with your registration for, and use of, the E-mail Program may include your e-mail address and elements of pharmacy claim information, such as name, date of birth, and prescription information. You agree that such data may be used to administer the E-mail Program and to provide E-mail Program benefits such as savings offers, information about your prescription, including refill reminders and new prescription requests, as well as E-mail Program updates and alerts sent directly to your e-mail address.
You may unsubscribe from the E-mail Program at any time by clicking on the unsubscribe link at the bottom of any E-Mail Program e-mail. Please do not reply to E-mail Program e-mails as it is an unattended e-mail box. A link to contact Viatris is at the bottom of every E-mail Program e-mail. Viatris reserves the right to rescind, revoke, or amend the E-mail Program without notice.
YOU ARE LEAVING LYRICA.COM, A VIATRIS INC., COMPANY WEBSITE
Links to another site are provided as a convenience to the viewer.
YOU ARE LEAVING LYRICA.COM, A VIATRIS INC., COMPANY WEBSITE
The information provided in LyricaHCP.com is intended only for healthcare professionals in the United States.